Technical sheet
TÜV CERTIFIED* AND HEALTH CANADA cleared medical device
Universal Power Supply:
120/230 V~ 60/50 Hz
Fuse Type:
T1.5 AL (120/230 V~)
Applied Parts Degree of Protection:
Type BF
Operating Mode:
Continuous
Current Consumption:
1.0 A
Protection Class:
1 (Grounded)
Tolerance / Accuracy of display:
± 15 %
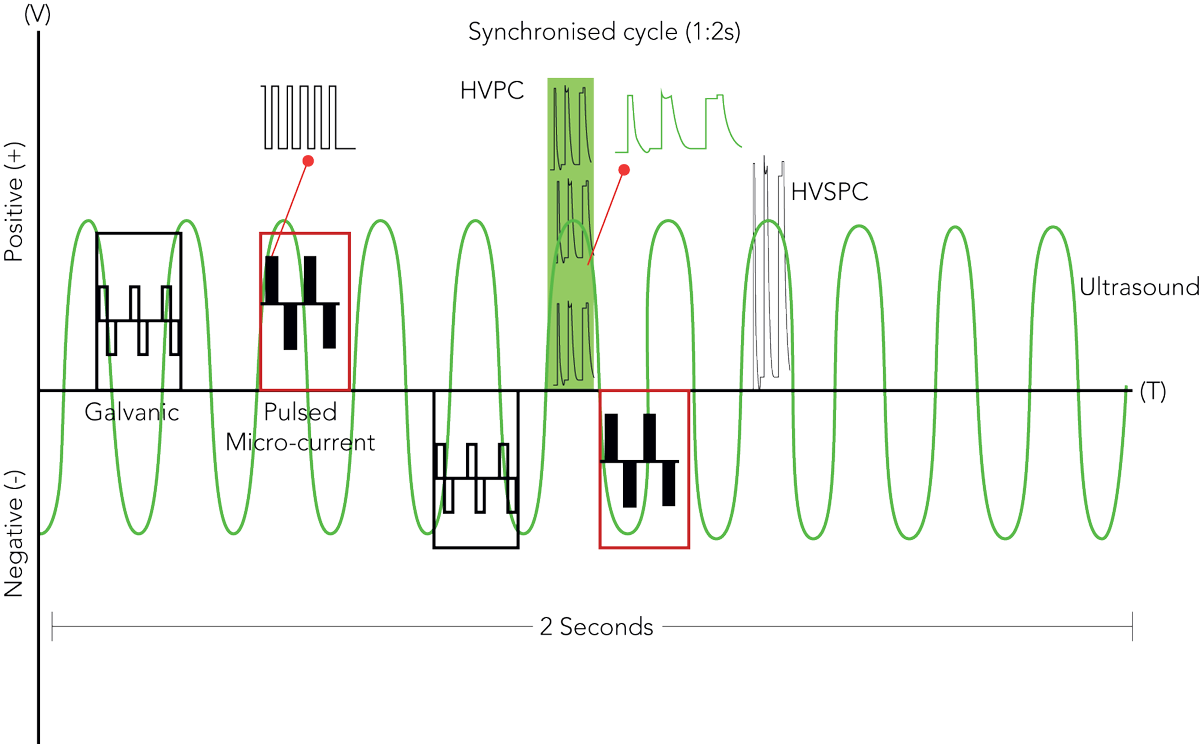
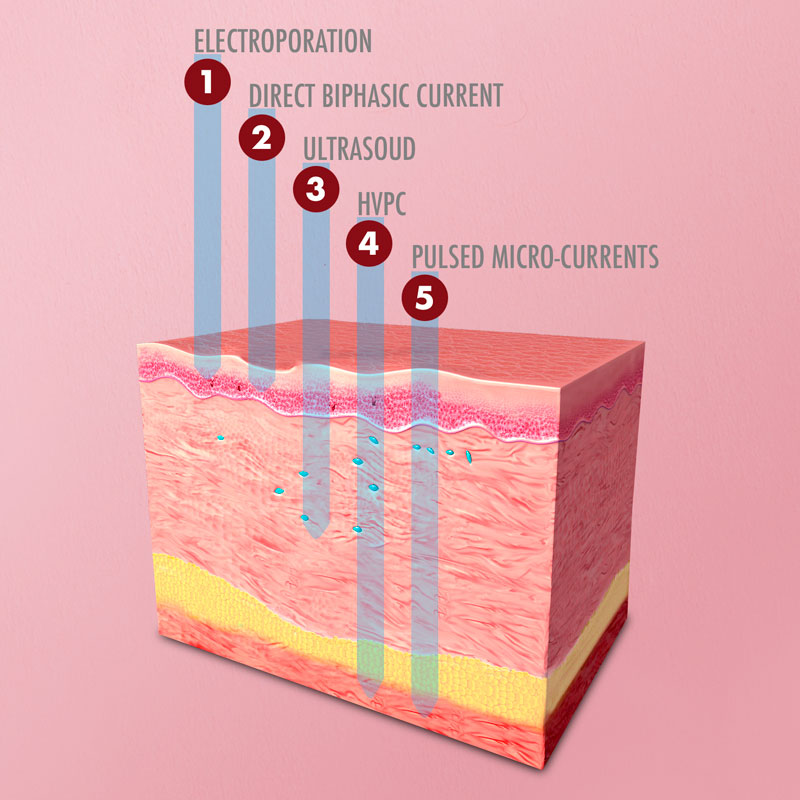
Acoustic Output Frequency:
3 MHz continuous sine wave
Maximum Acoustic Output Power:
4.32 W
Maximum Acoustic Power Density:
0.35 W/ cm
Effective Radiating Area:
12.5 cm
BNR:
0.07
Beam Type:
Divergent
Water-ingress Rating:
IPX7
Maximum HVPC Signal Amplitude:
135 V p-p
HVPC Signal Durations:
60-90-120 µs
Maximum Micro Currents:
3000 µA
Micro Currents Carrier Frequency:
187.5 Hz
Unit Dimensions:
Width: 15 in (381 mm)
Height: 9 in (229 mm)
Depth: 21 in (535 mm)
Unit Weight:
10 lb (4,5 kg)
Equipment Construction:
Internal steel frame
Kydex body
Electronics:
Microprocessor controlled
Hand-held Accessories:
Piezoelectric actuator
Ambient Operating Temperature:
+10°C to +40°C
Ambient Operating Humidity:
30% to 75% HR
Ambient Operating Atmospheric Pressure:
700 hPa to 1060 hPa